Innovative technology for the production of functional and safe human colonic microbiota for microbial transplantation - ETHIRA-Grant
The human gut is the natural habitat for a large, diverse and dynamic bacterial community with important functions for its host, including developmental, metabolic, protective and trophic functions. Microbial perturbations of this complex ecosystem have been reported in many diseases including chronic infections, inflammatory bowel diseases, diabetes, malnutrition and obesity. Recent research have suggested that fecal microbiota transplantation holds therapeutic potential to restore healthy microbiota, such as for the treatment for recurrent Clostridium difficile infection, with success rate greater than 90% and no adverse events. However, application of this ground-breaking treatment is bound to donor identification and selection, safety, aesthetic and ethical considerations.
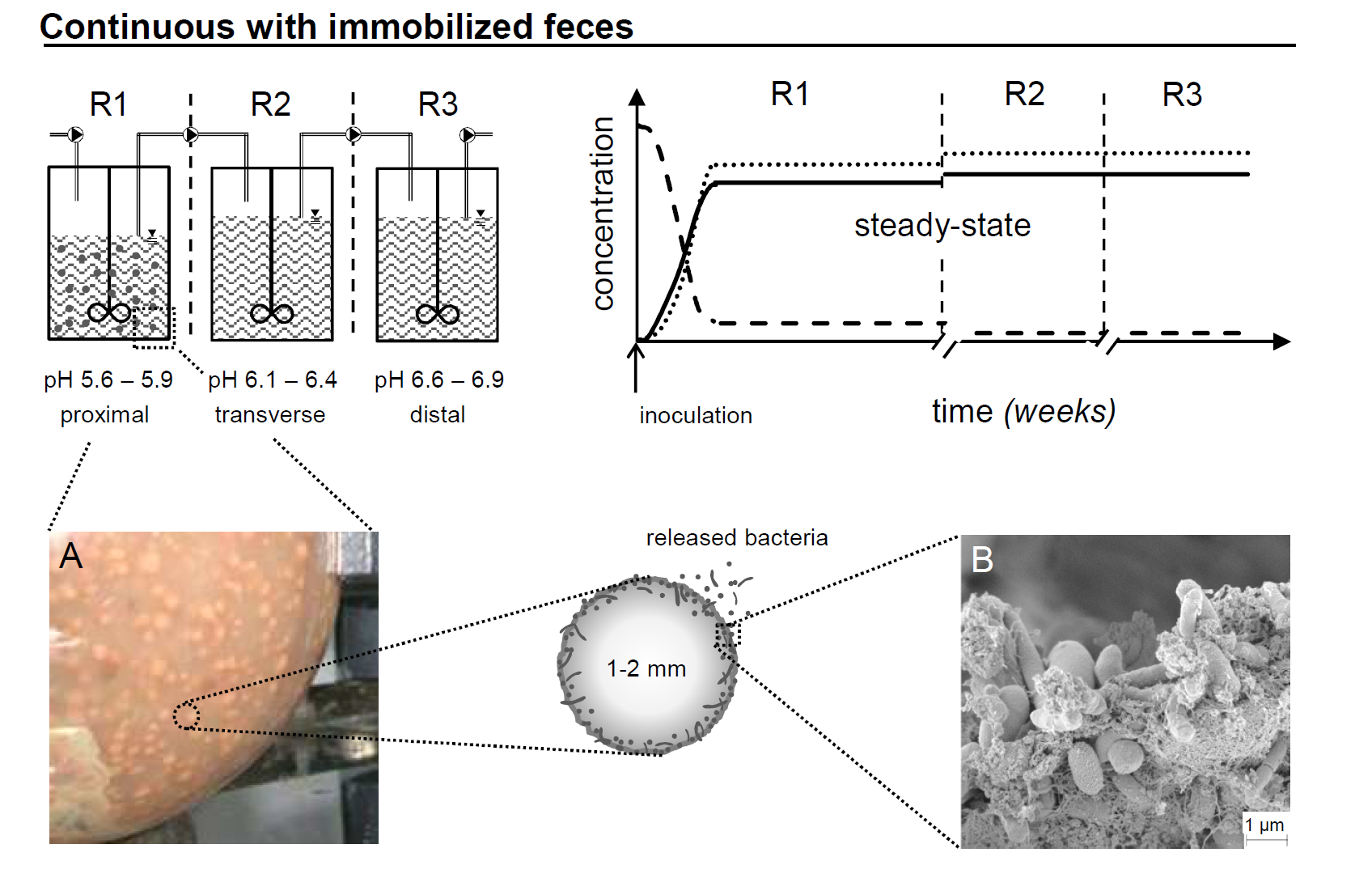
In this project we aim to set and validate a novel safe and efficient technology for the production of colonic microbiota for controlled and safe intestinal microbiota transplants (IMT). Our fermentation technology is based on the PolyFermS platform for continuous intestinal fermentation, using immobilized fecal microbiota in polysaccharide gel beads. Biodiversity, functionality and stability of artificial human colonic microbiota produced in vitro are in depth investigated during extended fermentation periods, and using state of the art methods (16S rRNA amplicon sequencing, meta-transcriptomics). Factors for modulation of composition and activity of the microbiota are investigated. Conditions for downstream processing, stabilization and frozen storage of “artificial”-produced gut microbiota are also studied, including optimal fermentation, cell conditioning, and development of protective matrices.